-
PDF
- Split View
-
Views
-
Cite
Cite
Amit Chakraborty, Bai-Lian Li, Departure from naturalized to invasive stage: a disturbance-induced mechanism and associated interacting factors, Journal of Plant Ecology, Volume 3, Issue 4, December 2010, Pages 231–242, https://doi.org/10.1093/jpe/rtq021
Close - Share Icon Share
Abstract
Within a habitat of multiple plant species, increased resource availabilities and altered species abundances following disturbances create opportunities for exotic species to successfully establish and subsequently naturalize into its non-native environment. Such post-disturbance changes in abiotic and biotic environments may also promote a naturalized exotic species (or invading species) to become invasive through rapid colonization of the habitat sites by reducing the extent and size of resident plant species. By combining species life history traits with that of the disturbance-induced changes in habitat characteristics, we aimed to determine those interacting factors and associated mechanism allowing an exotic invasion to start off.
We used a modified version of the classic competition–colonization (CC) model which was formulated first by Hastings (1980) and studied later by Tilman (1994) to explain spatial coexistence of multiple species. Within this model framework, recruitment-limited spatial competition has explicitly been linked with interspecific resource competition without altering the basic assumptions and structure of the original CC model.
The model results showed that at a constant rate of resource supply, invading species can stably coexist with native species via trade-offs between species competitive ability and colonizing ability. On the other hand, the model predicted that with a fluctuating resource condition, invading species can successfully invade a habitat following continuous reductions in the size and extent of native species. Whether or not invading species holds competitive superiority over the native species for limiting resource, we showed that there exists a range of variation in available resource that allows an exotic invasion to start off in post-disturbance habitat. The associated disturbance-induced mechanism promoting invading species to become invasive has been identified. It states that occurrences of disturbances such as fire or clear-cutting influence variation in resource availability, and in addition open up many vacant microsites; given these disturbance-induced changes, invading species with a higher rate of propagule production and with a higher survival rate of adults particularly in low-resource condition recruits microsites at faster rate relative to native competitor species, and with a given range of variation in resource availabilities, it maintains continued expansions following reductions in size and extent of native species. Moreover, we identified those interacting factors and their specific roles that drive this mechanism. These factors include propagule supply, variable resource level and vacant microsite availability. Increased availability of vacant microsites following disturbances creates an opportunity for rapid colonization. Given this opportunity, higher number of propagules supplied by the invading species enhances the rate of colonization success, whereas the resource variation within a range of given thresholds maintains enhanced colonization rate of the invading species while it depresses native competitor species. Owing to the each factor's invasion regulatory ability, controlling one or all of them may have strong negative impact on the occurrence of exotic invasion.
INTRODUCTION
The process of exotic species invasion involves multiple successive stages, and for an introduced species to become invasive in a foreign habitat, it must overcome all the preceding stages (Vermeij 1996; Theoharides and Dukes 2007). Williamson and Brown (1986) provided a rough estimate of introduced species that become invasive. According to this quantitative estimate based on European plant data, only 10% of introduced species become ‘naturalized’ (i.e. form a self-sustaining populations) and only 10% of naturalized species become potentially ‘invasive’ (i.e. able to invade and reduce the size and extent of native species). Except those imprecise numbers, a general implication of these estimates has later become renowned as ‘tens rule’ which states that only a few invasions actually become successful (Williamson 1992, 1996). Although it deviates among many regions and taxa (Lodge 1993a; Williamson 1996), as a predictor of invasion success, the tens rule has held true for a variety of organisms, including angiosperms, grasses, legumes, terrestrial vertebrates, fishes, mollusks and plant pathogens (Lodge 1993a, 1993b; Mills et al. 1993; Williamson 1996). Subsequently, many invasion studies suggest that exotic species can rarely be excluded once they naturalized (Blumenthal et al. 2009; Milbau and Stout 2008). During naturalization, through the acclimation into foreign environment, exotic individuals successfully establish at several locations or habitat sites and then start reproducing without human intervention. Afterward naturalized individuals form small subpopulations that may be tightly linked through colonization (Melbourne et al. 2007), and the total population size is mostly constrained by intra- and interspecific interactions and the environmental conditions in which they interact with natives. These interactions are characterized as local ‘biotic filter’ that forms a barrier to invasion (Davis et al. 2000; Dukes and Mooney 1999). In contrast, at the invasive stage, invading species starts spreading in concurrence with continuous reductions in the extent and size of native species. A general spreading pattern at local spatial scale looks like a linear rate of expansions that are stemmed from many small invasion foci, though the rates vary among species depending on the dispersal mechanism (Arim et al. 2006; Morozov et al. 2008; Pyšek and Hulme 2005). At regional scale, the spread are resulted from these steady local spreads and rare long-distance dispersal events which usually tend to make the spread rate non-linear (Hastings et al. 2005; Lewis and Kareiva 1993; Neubert and Caswell 2000). These noted differences between the two stages of exotic invasions and other numerous observations across regions and taxa suggest that an ‘intermediate phase’ takes place between the ‘naturalized’ and ‘invasive’ stage of exotic invasion (Richardson and Pysek 2006; Theoharides and Dukes 2007), holding ‘naturalized’ stage for a period of time. This phase may correspond to lack of genetic variation that prevents rapid adaptation to novel conditions (Sakai et al. 2001) or the time necessary for the population to evolve with superior competitive abilities that allow it to spread following continuous reductions in size and extent of native competitor species (the evolution of increased competitive ability hypothesis) (Blossey and Notzold 1995). At ecological time scale, this intermediate phase may also reflect a lack of favorable local habitats or a period of severe disturbances (Pyšek and Hulme 2005). For example, while reviewing tree invasions into old fields, Myster (1993) emphasized the importance of a favorable local habitat that periodically ‘emerge’ during a wet year and ‘diminish’ during a drought year. A favorable local habitat that promotes invading species to departing from ‘naturalized’ to ‘invasive’ stage will be termed here as ‘switching point’ or ‘departure point’. A departure point allows invading species to efficiently make use of its inherent invasive abilities to invade and reduce the extent and size of native species. Its characteristics are determined by the invasive abilities of invading species (‘species invasiveness’), the structure of native community and the factors of community invasibility making the community susceptible to invasion (e.g. fluctuating resource availabilities). In absence of departure point, invading species may remain as naturalized, coexisting with natives for a long period of time without causing significant damage to its native competitor species. One steady example in support to its natural existence comes from black cherry, Prunus serotina, a native to North American forests and introduced in European forests for ornamental, timber production and soil amelioration purposes during 17th century. It was considered as naturalized in the first half of the 19th century but described as an invasive species only in the second half of the 20th century (Starfinger 1997). Kopp et al. (2007) revealed that P. serotina successfully invades European forests through a combination of traits that fits well to the disturbance-induced gaps that occurred frequently. The idea of departure point is also consistent with the established notion that many successful invasions can most likely to be occurred in areas of high-resource availabilities or under fluctuating resource conditions (Davis et al. 2000; ; Leishman and Thomson 2005; Tilman 2004). Temporal heterogeneity in resource availability may open short windows of high-resource availability that drive successful colonization by invading species; however, during the periods of low-resource availability, the earned benefits can be forfeited by the natives with higher competitive abilities. Thus, the invading species can capitalize and retain that short benefits over times while a suite of traits allows them to withstand strong competition from natives at low-resource conditions, leading to depart from naturalized to invasive stage (this issue). The idea also holds a parallel view of the concept of ‘niche opportunity’—that the some kinds of opportunities must be made available to an invading species for becoming invasive. However, both the ideas exhibit marked differences in underlying mechanisms and explanations that they provide for invasion success. The niche opportunities such as enemy escape or resource opportunities are determined by the degree of niche overlap between interacting native species and the physical conditions in which they interact; opportunities are made available to an invading species by an extrinsic factor that lessens niche overlap or by the intrinsic effect of species on physical environment (Shea and Chesson 2002). It strongly emphasizes species invasiveness that allows invading species to use such opportunities to make the invasion successful. It, however, does not provide any insights on how momentary or periodic benefits can be capitalized and retained to maintain invasion success over time in variable environment. The idea of departure point particularly fills this knowledge gap. As we illustrate here, at the departure point an invading species holds a combination of traits that fits well with the opportunities available and simultaneously it allows retaining the benefit over time in a variable environment. Owing to the exclusive dependence on the fine matching between species invasiveness and the types and levels of opportunities available for invasion, the departure point may likely to be infrequent in natural community. Such highly infrequent occurrences of departure point may provide a potential explanation for ‘tens rule’ which states that successful invasions are relatively rare in nature.
While viewing disturbances as a source of increased resource availabilities through the creation of open ground by removal of individuals or through the enhanced resource supply (Brokaw 1985; Collins and Barber 1985; Collins 1987; Hobbs and Huennek 1992; Hobbs and Mooney 1985; Platt 1975), the proposal of Davis et al. (2000) that a plant community becomes more vulnerable to invasion with an increase in the amount of unused resources provides a link between disturbances and invasibility of a plant community. The link rests on the assumption that invading species must have access to disturbance-mediated resources, and it will enjoy greater success in invading a community if it does not encounter intense competition for these resources from native species. Depending on the inverse correlation between competition intensity and amount of unused resources, the proposal strongly indicates a positive relationship between disturbance and invasive potential of plant communities (Davis et al 1998). This understanding of exotic invasion provides a basis to the general notion that the disturbances facilitate invasion, and it has strongly been supported by a large number of field observations and experimental evidences (Burke and Grime 1996; Crawley 1987; D'Antonio 1993; Elton 1958; Hobbs 1989; Hobbs and Atkins 1988; Hobbs and Mooney 1985; Huenneke et al. 1990; Lodge 1993b; Stohlgren et al. 1999; Symstad 2000). However, comparative ecological studies of invading species where they are native versus where they are exotic suggest that disturbances alone cannot explain invasion success (Hierro et al. 2006). Given the ruderal life history of many invading species (Baker 1974; Grime 1974), it is highly expected that they flourish in disturbed habitats. If, however, disturbances are sufficient to explain exotic invasion, then the invading species with ruderal strategy should respond similarly to disturbances where they are native. But this is not always the case as evident in an experiment by Hierro et al. (2006), they showed that disturbances have much stronger effects for Centaurea solstitialis invasions into the non-native ranges in California and Argentina than its native ranges in Turkey. Now, the modern approaches in invasion ecology embrace the idea that successful invasions are driven by the disturbances in association with other factors of exotic invasions such as propagule pressure (Didham et al. 2007; Eschtruth and Battles 2009; Kellogg and Bridgham 2004; Richardson and Pysek 2006; Rouget and Richardson 2003; Lake and Leishman 2004; Theoharides and Dukes 2007). With these modern approaches, further decomposition of disturbance effects into temporal (e.g. temporal heterogeneity in resource availability) and spatial components (e.g. vacant habitat sites) allows for a more detailed analysis of exotic invasion, which has rarely been examined. We illustrate here how such disturbance-mediated temporal and spatial effects influence switching from ‘naturalized’ to ‘invasive’ stage at the departure point.
In this paper, we characterize and describe a departure point within a habitat of multiple plant species and then illustrate how disturbance-induced changes in resource availabilities and vacant spaces influence invading species and eventually lead to switching from ‘naturalized’ to ‘invasive’ stage. To do so, we analyze a modified version of the classic competition–colonization model that was formulated first by Hastings (1980) and studied later by Tilman (1994). Within the framework of the original model, colonization by species is represented as a local recruitment process which is constrained by interspecific resource competition and that of species’ inherent abilities to disperse and produce propagules. This model is particularly appropriate for addressing such issues because of its strict hierarchical structure based on species competitive abilities and involved trade-offs between competitive and colonizing abilities. Together these features facilitate characterization of a departure point by outweighing the effect of competitive hierarchy via the disturbance-induced changes. The ‘naturalized’ stage of an invading species is reflected in the model with its specific competitive ranking that allows coexistence with natives for an indefinite period of time in absence of disturbances (Chakraborty and Li 2010; Tilman 1994). It can also be mentioned that the model has shown to be useful for illustrating some of the core concepts in community ecology such as limiting similarity, species packing and system stability (Kinzig et al. 1999; Morozov and Li 2008). Besides its theoretical impetus, the model has also been applied to examine species loss under habitat destruction in high-diversity tropical forest systems of tens to hundreds of species (Tilman et al.1994).
MODEL AND METHODS
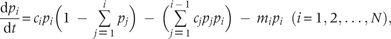
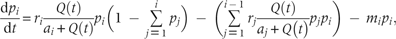
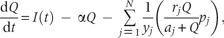
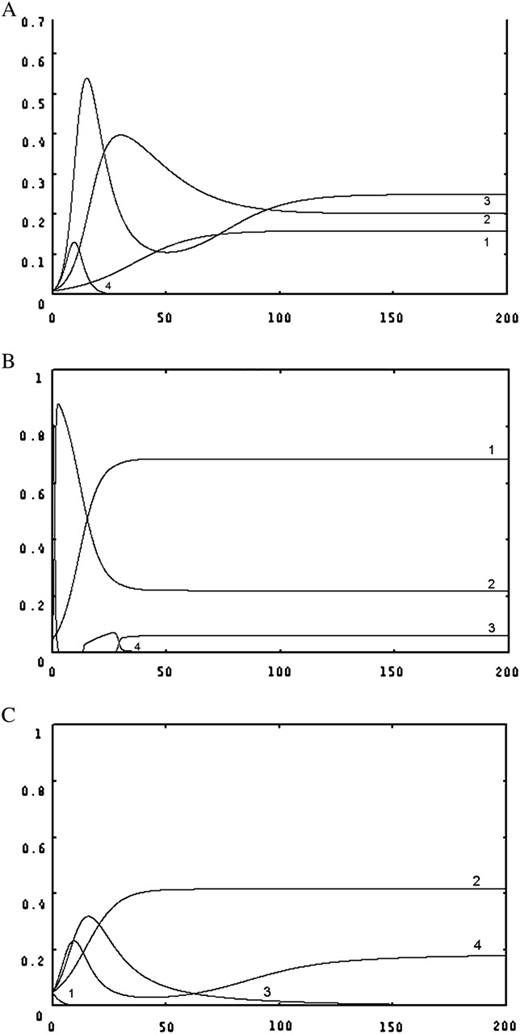
While there is no resource limitation, species recruitment rate is exclusively determined by the intrinsic rate of propagule production (i.e. as ). In this situation, the modified model has retained the same properties as the original CC model. There exists a limit to similarity between species—that a minimum value for this recruitment rate termed as ‘niche shadow’ by Kinzig et al. (1999), which allows stable coexistence of superior and inferior competitor species. If species satisfy this condition, the model predicts coexistence of unlimited number of species, i.e. there is no limit to the number of coexisting species (Tilman 1994). While available resource is limited, the modified model predicts a limit to this number, where the limit to similarity between species has remained unchanged ( Appendix). It describes that at a constant rate of resource supply, the competing species reach at an equilibrium point irrespective of their initial abundances (Fig. 1) in which they partition the space according to their equilibrium recruitment rate, ci, such that both R* and B* requirements are fulfilled by the habitat's resource concentration (i.e. ). Thus, in this case, the resource quantity defines a limit to the number of coexisting species.
spatial dynamics of four competing species in a constant environment, with species of rank 1 is the best competitor but poorest colonizer whereas the species of rank 4 is the best colonizer but worst competitor (A) all species have the same relative rates of propagule production (ri = 0.5 yr−1, i = 1, 2, 3, 4) but have different mortality rates (m1 = 0.4, m2 = 0.225, m3 = 0.01, m4 = 0.02 yr−1), different half-saturation coefficients (a1 = 0.5, a2 = 0.6, a3 = 0.7, a4 = 0.8 kg ha−1), different yield coefficients (y1 = 0.8, y2 = 0.9, y3 = 0.9, y4 = 0.5). The resource supply rate (I) was maintained at 15 kg ha−1 yr−1 and the resource loss rate (α) was fixed at 1.5 kg ha−1 yr−1. All the species have the initial abundances of 0.01. (B) All species have the same mortality rates (mi = 0.1 yr−1, i = 1, 2, 3, 4), but have different relative rates of propagule production (r1 = 0.333, r2 = 3.7, r3 = 41.15, r4 = 157.2 yr−1), different half-saturation coefficients (a1 = 0.5, a2 = 0.6, a3 = 0.7, a4 = 0.8 kg ha−1), different yield coefficients (y1 = 0.1, y2 = 0.05, y3 = 0.2, y4 = 0.6 kg−1). The resource supply rate (I) was maintained at 20 kg ha−1yr−1 and the resource loss rate (α) was fixed at 1.5 kg ha−1yr−1. All the species have the initial abundances of 0.05. (C) All species have the different relative rates of propagule production (r1 = 0.1, r2 = 0.35, r3 = 0.4, r4 = 0.5 yr−1), also have different mortality rates (m1 = 0.4, m2 = 0.2, m3 = 0.1, m4 = 0.05 yr−1), different half-saturation coefficients (a1 = 0.08, a2 = 0.7, a3= 0.8, a4 = 1 kg ha−1), different yield coefficients (y1 = 0.1, y2 = 0.05, y3 = 0.2, y4 = 0.6 kg−1). The resource supply rate (I) was maintained at 50 kg ha−1yr−1 and the resource loss rate (α) was fixed at 1.5 kg ha−1yr−1. All the species have the initial abundances of 0.05.
Tilman's R* rule suggests that while species compete for a single limiting resource in a homogenous environment, the best competitor excludes others by depleting the resource to a lowest level at which other competitors cannot survive (Tilman 1982). This, seemingly a robust finding, has formed a basis for the hypothesis that multiple competing species coexist by habitat heterogeneity (Chesson 1986; Comins and Noble 1985; Grubb 1977; Levin 1974; Pacala and Tilman 1993; Tilman 1982). While introducing propagule-limited recruitment with that of the trade-off between species competitive ability and recruitment ability, the modified CC model predicts coexistence of multiple competing species in a homogenous resource environment. Here, the species competitive ability is considered in a strictest sense that an ability to deplete the resource to a lower level than its competitor species. Specifically, in this study, R* value refers to a lowest resource level that can be depleted by a species. As the competition success cannot secure long-term persistence in a habitat, in order to persist in a resource-poor condition, a species must support recruitment through propagule production in expense of reduction in resource use for survival in resource competition. Here, the B* value refers to a minimum resource level that allows to continuing this recruitment process. Given the inverse relationship between R* and B*, a best competitor may not adopt a competition strategy that allows depletion of habitat's resource level to its R* value because it strongly buffers its microsite recruitment. Mathematically, this situation can be explained by the condition, ; at an equilibrium, cannot be equal to Q* because implies . With the interspecific trade-offs between competitive ability and recruitment ability coupled with the condition, , a superior competitor therefore not excludes its inferior competitors. As a result, an inferior competitor can stably coexist at equilibrium by manipulating its equilibrial abundance such a way that its B* value remained below the habitat's resource concentration, which is reflected in our calculation of Q* included in Appendix.
SPECIES INVASION IN A VARIABLE ENVIRONMENT: A DEPARTURE POINT
Without disturbances we envision coexistence of naturalized exotic species (or invading species) and native species in the system described by the equations (2) and (3). While disturbances occur, such as fire or clear-cutting, there may have strong effects on the level of available resources because disturbances often release resources or alter the rate of resource supply (Carson and Pickett 1990). Moreover, disturbances usually open up many vacant microsites. We incorporate both these situations into our analysis. Changes in resource supply and the subsequent effects on resource variability would prevent the interspecific competition from reaching at stable equilibrium point. In the previous section, we have shown that at a constant rate of resource supply each competitor species approaches toward an equilibrium point irrespective of their initial abundances. On the other hand, availability of many vacant microsites reduces the effect of microsite-limitation on the rate of production of newly recruited microsites (the first term of the equation 2); thus in this situation, this rate primarily depends on the variable resource level and on the species characteristics: the intrinsic rate of propagule production (ri) and half-saturation coefficient (ai).
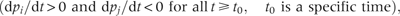
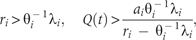
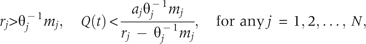
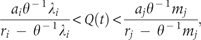
The departure point is determined by two distinct species traits: the specific species trait, , will be called ‘invasive trait’, determines the lower resource threshold, and the another species trait, , will be called ‘native trait’, determines the upper resource threshold. The first one differs from the second because of the presence of an additional term . The invasive trait is jointly influenced by θ−1 and λ, reflecting combined effects of available vacant microsites and competitive hierarchy. As the value of θ depends on the number of vacant microsites, more microsite availability refers to higher θ-value and thereby higher reduction in the combined effect. In turn, this reduction reflects lowered competition intensity from native species during spatial expansion of invading species.
The above derivation shows that primarily two distinct habitat factors, strict competitive hierarchy and availability of vacant microsites, affect the invasive and native traits relating to exotic invasion. With the invasive traits, invading species embraces significantly higher intrinsic rate of propagule production relative to its native superior competitor species and that represents the primary characteristic that distinguishes putative invasive species from native species. There is no effect of competitive hierarchy if an invading species holds a competitive rank one. We consider two different cases while an invading species holds a competitive rank, >1; its native competitor species j (j = 1, 2, …, N) could have the competitive ranking either >i or <i.
Case-1: The invading species i is competitively inferior to the native species, j (j = 1, 2, …, i−1), i.e. i > j: the conditions under which the departure point (7) is maintained by the invading species are,
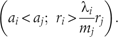
The first term within the brackets is implied from the fact that because of i > j, species i holds lower -value relative to the native species j (considering proportionality between and ai). In the second term, because , and j is some of (1, 2, …, i − 1). The second term indicates significantly higher intrinsic rate of propagule production than its native competitor species j. Therefore, the specified range of variation in available resource (equation 7) allows a competitively inferior invading species with a higher intrinsic rate of propagule production to outperform competitively superior native species.
Case-2: The invading species i is competitively superior to native competitor species j, i.e. 1 < i < j (j = i + 1, i + 2, … N): the following conditions allow the invading species to maintain the departure point (7):
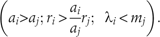
Since i < j, species i holds higher -value relative to native j, which implies the first term within the brackets. The second term represents higher intrinsic rate of propagule production, and the third term indicates higher survival rate of species i. Thus, the specified range of variation in available resource allows a competitively superior invading species with a higher rate of propagule production and survival to outperform competitively inferior native species.
A successful invading species can be a worst competitor relative to all native competitor species, which follows from the case-1. While an invading species holds the competitive rank one, its abundance dynamic is not affected by competitor species; in this case, the invasion condition (9) has remained the same except the third term in which is needed to be substituted by . In general, we found that a naturalized exotic species with a higher intrinsic rate of propagule production and with a higher survival rate of adults can become invasive irrespective to its competitive ranking. These traits combined with the variation of resource level within the range of given thresholds allow an exotic invasion to start off in post-disturbance habitat.
RESULTS AND DISCUSSION
General characteristics of a departure point
At a departure point, naturalized exotic species (or invading species) departs from ‘naturalized’ to ‘invasive’ stage. The associated departure dynamics exhibit continuous increases in population size and spatial extent of invading species following reductions in size and extent of native competitor species. The spread or range expansion represents a monotonically increasing function of a population that stemmed from a single invasion foci and will later spread in all directions. For example, Foxcroft et al. (2004) estimated that over a period of 50 years, one population of Opuntia stricta spread up to 18.5 km from its origin at an average rate of 370 m yr−1. The related characteristics of a departure point depend on the species abilities to invade and withstand competition from natives, the factors of community invasibility that make a community susceptible to invasions and the type and structure of native community. Using the classic CC model, we described a departure point that involves disturbance-meditated vacant areas that present direct colonization opportunities to all competing species. It also involves ‘resource opportunity’ (Shea and Chesson 2002) arising from fluctuation in resource availability; the resource fluctuation weakens competition, resulting in increased amount of unused resources (Davis et al. 2000). Given both the opportunities, invasion occurs along a continuum of resource heterogeneity (equation 7). The one end point of the continuum represents an invasion threshold with a minimum biotic resistance to invasion resulting from weak competitive interactions. At this point, invading species act on both the opportunities available to its population and start-off spreading with an elevated rate of population growth. The levels of the opportunities, i.e. the amount of unused resource available and the number of opened microsites depend on the frequency and severity of the disturbances experienced at the point. So, as a start-off point for an invasion to occur, the rate of invasion exhibits an increasing propensity with the highest levels of available opportunities. The other end point of the continuum represents a ‘biotic resistance threshold’ that allows an invading species to invade and reduce the size and extent of native competitor species. At this point, both the opportunities are minimized but still accessible with the levels that support an increasing rate of invasion. At this end, invading species will capitalize and retain the prior benefits of enhanced abundance occurred during the period of highly reduced biotic resistance and eventually will outperform native competitor species as they fail to skew the expansion rate. If the temporal fluctuation in resource availability keeps the resource level little above the invasion threshold, the invasion rate will be maximized with rapid reductions in size and extent of native species. On the other hand, if the temporal fluctuation in resource availability keeps the resource level below but near to the biotic resistance threshold, invasion rate will be relatively slowed with a decreased rate of reductions in the extent and size of native species. Nevertheless, throughout this continuum, temporal resource heterogeneity allows an invading species to increases continuously in its population size and spatial extent following reductions in the size and extent of native species. As we discussed in the following section, this invasion scenario must embrace a combination of invasive traits that allows an invading species to colonize rapidly during the period of high-resource availability and simultaneously withstand competition from natives during the period of relatively lower resource availability. These characteristics of a departure point parallel to the available case histories of invading plant species such as Senecio squalidus, Buddleja davidii, Impatiens glandulifera and Heracleum mantegazzianum. It indicates that these species have mostly invaded habitats with linear features such as riverbanks, roadsides and railways. While these corridors have provided access to invading species through their continuity and relatively high-resource availabilities, a high level of disturbances such as flooding, burning, herbicide treatments, clear-cutting has facilitated colonization along such conduits by creating open areas of bare soil (Grime 2001).
Species invasiveness exhibits weak trade-offs between r- and K-strategy at a departure point
The model results suggest that it is essential for successful invasion to have a fine matching between the levels of both vacant space and resource opportunities and the invasive abilities that enable invading species to efficiently utilize them. A combination of invasive traits that matches well with these opportunities includes a higher rate of propagule production coupled with a higher survival rate of adults. A higher propagule production rate has strongly been favored when both the opportunities available with higher degrees, leading to enhanced rate of recruitment. When the opportunities are limited, higher survival rate allows invading species to withstand competition from natives. Particularly in low-resource environment, species those possess higher survival rate commonly express traits associated with resource conservation such as a long leaf lifespan, thicker leaves or low tissue-nutrient content; according to resource allocation theory, such trait expressions reduce growth rate but maximizes carbon assimilation per unit of resource (Funk and Vitousek 2007; Noordwijk and Jong 1986). It, therefore, implies that given both a higher rate of propagule production in high-resource conditions and a higher rate of survival relatively in low-resource condition, invading species retains higher benefits over native competitor species throughout the range of the opportunities available at a departure point. This result is consistent with the outcomes of comparative analysis between the two invasive tree species, Ailanthus altissima and Acer platanoides, and the nine native tree species in closed-canopy forests in Connecticut. Martin et al. (2010) found that both A. altissima and A. platanoides had higher growth rates relative to all natives at high-light levels (>10% full sun); however, besides higher growth rate at high-light level, the highly invasive A. platanoides had the annual mortality rate <1% at 5% full sun which is significantly a lower mortality rate than the most dominant natives.
Classification of species based on the r-K selection continuum, an ideal ecologic vacuum, relies on the fundamental life-history trade-offs between reproduction and survival. Species that allocate most resources into reproduction and produce as many total progeny as possible (r-strategy) cannot withstand competition from species those allocate most resources into maintenance and produce a few but extremely fit offspring (K-strategy). As an ecologic vacuum is filled with species, natural selection will favor K-strategist over r-strategist species (MacArthur and Wilson 1967). While the r-end point of the vacuum represents an extreme with no competition and no density effects, the K-end point represents another extreme with highest density effects and strong competition. Certainly, no organisms purely possess r- or K-strategy, rather they are either inclined toward r-end point or K-end point (Chakraborty and Li 2009; Pianka 1970). While an invading species embraces both a higher rate of propagule production at high-resource condition and a higher rate of survival at low-resource condition, it exhibits a strong compromise between these two extremes, and that allows it to hold an intermediate position along the ideal ecologic vacuum. Recently, Kopp et al. (2007) reported a similar synthesis for P. serotina, a successful invading tree species in European forests as the P. serotina exhibits both a superior survivor in shades and a superior colonizer in gaps.
Interactions between disturbance-induced changes and propagule supply promote departure from naturalized to invasive stage
The model results have identified three potentially interacting factors that drive invasions in post-disturbance environment. These factors include availability of vacant microsites, propagule supply and variable resource level. Each of these factors has the invasion regulatory ability. Among them, ‘propagule supply’ and ‘variable resource level’ have already received considerable attention from invasion ecologists (D'Antonio et al. 2001; Davis et al. 2000; Holle and Simberloff 2005), whereas the influence of the other factor, ‘availability of vacant microsites’, is relatively less appreciated. The present study identified their specific roles leading to departure from naturalized to invasive stage. Availability of many vacant microsites following disturbances creates colonization opportunity which is further enhanced by the fluctuating resource level that reduces competition intensity from native species. Given this opportunity, ‘propagule supply’ enhances the rate of colonization success. This supply can be human transported or by the invading species itself or both. The first one is discrete in nature and its role in species invasion has already been discussed in details in several research articles (Lockwood et al. 2005 and references therein). In contrast, the species-driven propagule supply is continuous within a given time frame, and its role in exotic invasions is relatively less obvious because its complex interactions with other factors. Here we demonstrated one such case in which it interacts with the other two invasion factors (Fig. 2). As shown in our results, with a higher intrinsic rate of propagule production coupled with a higher rate of survival of adults, invading species can supply more propagules into a habitat. In addition to its dependence on the invasive traits, its intensity (the number of propagules supplied into a habitat) also depends on the level of available resource and on the population density or size of invading species. As the density increases, the number of supplied propagules also increases, whereas the resource level restricts the supply by limiting the intrinsic rate of propagule production (equation 2). Thus, the temporal resource variation can alter the number of propagules supplied into a habitat. On the other hand, the resource variation can play another important role to start-off an exotic invasion: it can relax the propensity of stable species coexistence. Otherwise, without resource variation, all the competing species may approach toward a stable equilibrium point in which they stably coexist by partitioning the space corresponding to their equilibrium abundances, as predicted by our model. Depending upon the nature of resource variation, there may have multiple conditions for an invasion to occur (Schoolmaster and Snyder 2007). Here, we found one such condition—the upper and lower resource thresholds. This condition allows a naturalized exotic species to become invasive regardless of its competitive ranking.
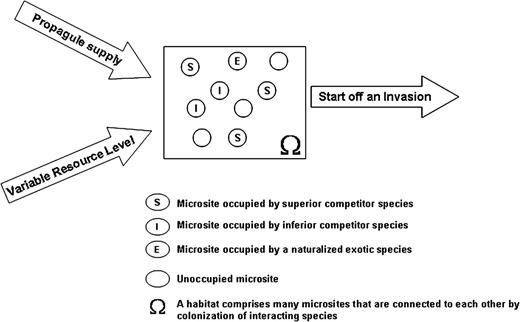
a diagram illustrating an invasion condition: a naturalized exotic species (termed as invading species) becomes invasive when there are changes in habitat characteristics through the interactions among the factors of propagule supply, variable resource level and availability of vacant microsites. A higher intrinsic rate of propagule production combined with a higher survival rate of adults allows an invading species to supply more propagules into a habitat. Availability of many vacant microsites reduces the competition intensity from native species, whereas the variable resource level within the specified range maintains the increasing recruitment rate of invading species by depressing native species irrespective of its competitive ranking.
A mechanistic explanation for biotic resistance to invasion
One prominent hypothesis on community resistance to invasions is that a species-rich community is more resistant to invasion than a species-poor community (Elton 1958). However, results of experimental and observational studies often conflict, leading to debate about the importance of diversity in determining the occurrence of an invasion (Knops et al. 1995; Planty-Tabacchi et al. 1996; Rejmanek and Richardson 1996; Wiser et al. 1998). Our model results provide a mechanistic explanation underlying this hypothesis. Inclusion of species into a community intensifies the competition because it reduces the resource level and the number of vacant microsites that jointly affects the temporal patterns of species abundances as it described by the equations (2) and (3). Our analysis demonstrates that decreasing availability of vacant microsites elevates invasion resistance by enhancing the resource requirement for maintaining elevated population growth of invading species (equation 5). This enhanced resource requirement further intensifies the competition from native species and thereby raises strong resistance against invasion. Therefore, a joint effect of less microsite availability and lower resource level makes a species-rich community relatively more resistant to invasion than a species-poor community.
Finally, our goal in this paper has been to illustrate the influences of disturbance-induced changes on the occurrence of an exotic invasion. The work presented here offers a mechanism that involves interactions among the factors of propagule supply, variable resource level and vacant microsite availability, which may reconcile the importance of species life history traits and environmental factors for predicting future invasion, with the predetermination of CC trade-offs and the spatial scale at which the mechanism may apply.
FUNDING
US National Science Foundation's Biocomplexity Program (DEB-0421530), Long Term Ecological Research Program (Sevilleta) (DEB-0620482) and the University of California Agricultural Experiment Station.
We highly appreciated Edith Allen and her group for some useful discussions on this subject and Darrel Jenerette for commenting on the earlier version of this article. We also thank associate editor and two anonymous reviewers for their valuable comments that helped us to improve the quality of this article and clarify some of the important results with prior empirical and theoretical evidences.
APPENDIX
The qualitative behavior of the solutions of equation (2) shows that at a constant rate of resource supply, competing species reach at equilibrium one by one; once the best competitor attains its equilibrium abundance, the next equation for the next best competitor takes on the form of the first equation for the first species and the second species goes to equilibrium. This propagates down through N number of species independent of their initial abundances (Fig. 1). Following this trend, we can sequentially calculate habitat's resource concentration and the associated recruitment rate, starting with the species 1, in terms of equilibrium species abundances and other species-specific parameters. When ,
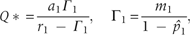
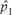 is the abundance of the species 1 and Q* is the habitat's resource concentration at equilibrium. The corresponding recruitment rate, c1, is given by the equation (2). Inserting c1 in the equation of , we obtain:
is the abundance of the species 1 and Q* is the habitat's resource concentration at equilibrium. The corresponding recruitment rate, c1, is given by the equation (2). Inserting c1 in the equation of , we obtain: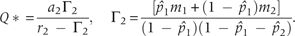
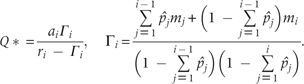
It could be noticed in the above equation that the calculation of Q* involves the effect of superior competitor species, and the Q* is defined for ri > Γi. Therefore, a species, species i, can persist in the system if and ri > Γi.
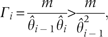
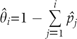 . Alternatively, can be described as,
. Alternatively, can be described as,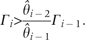
This relationship defines a limit to similarity between species, this limit is the same as it was calculated by Tilman (1994) using the original CC model.
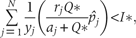
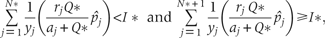
The above inequations imply that at a constant rate of resource supply, there exists a maximum N* number of species to coexist at equilibrium point.